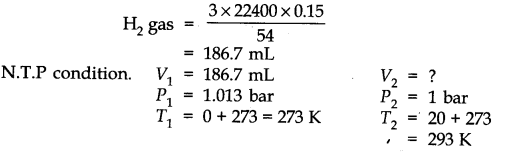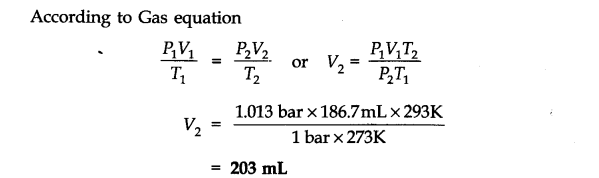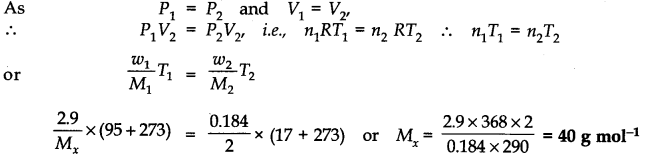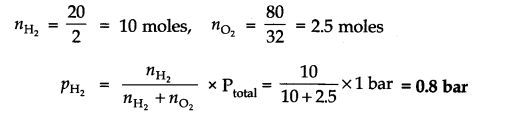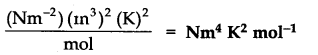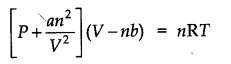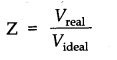NCERT Solutions For Class 11 Chemistry Chapter 5: NCERT Solutions For Class 11 Chemistry Chapter 5 is certainly one of the vital and complicated subjects to be included in the science stream. Therefore getting the subject to the core is sure to pave you a robust path for an endearing future. So, taking down only classroom instructions might not gift you the result that you aspire to achieve.
So, we are here with NCERT Solutions For Class 11 Chemistry Chapter 5 for your exam preparation. NCERT and CBSE are doing a great job in building the future of the young scholar from ages now. And more importantly, the NCERT follows that parameters that competitive exams generally abide by. Therefore going through the curriculum for 11th and 12th will do tremendous help altogether.
Also if you are planning to sit for JEE and NEET then taking preparation from class 11 is the only possible choice you have. Additionally, if you take your 11th standard seriously then there is a high chance of achieving an inspiring scorecard for your 12th boards.
NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter
The digital learning medium took a smart approach to strategize the academic backdrop. And now the internet swarms with various study material for you to take as a helping hand. Likewise, we bring you NCERT solutions for class 11 chemistry Chapter 5 pdf download from here.
Download class 11 chemistry Chapter 5 NCERT solutions pdf and start with your exam preparation right here right now. This smart application is not only easy but is efficiently convenient to make your study goal complete before the time.
Just have the content downloaded in your device and jump-start your exam prep in no time. Also, Hindi students make sure to have your copy of the same from NCERT 11th Chemistry Chapter 5 solutions pdf download in Hindi.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 5 from below.
NCERT Solutions For Class 11 Chemistry Chapter 5
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2021/07/chapter_5_states_of_matter.pdf”, “#example1”);
What will you learn in CBSE Class 11 Chemistry Chapter 5 State Of Matter?
State of the matter is basics that students start learning from class 4 itself. But with time the chapter adds section suiting cognitive progress of the children. Class 11 still offers rudimentary description while covering many essential topics such as the intermolecular forces and how they affect the physical state of a substance.
Additionally, the chapter also brushes over some vital topics related to the liquid and gaseous state of matter. Hence it is one of the important chapters that students of class 11 need to study by heart.
Types of questions that this chapter comes with-
- Arithmetic problem on Boyle’s law, Charles’s law, Gay-Lusscac’s law, and Avogadro’s law and lastly on the partial pressure.
- Then there will be questions on critical temperature, pressure, Van der Waals forces and other types of intermolecular forces.
CBSE Class 11 Chemistry Chapter 5 State Of Matter Subtopics
NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter Subtopics included in the chapter are as follows-
- Intermolecular Forces
- Dispersion Forces Or London Forces
- Dipole-dipole Forces
- Dipole-induced Dipole Forces
- Hydrogen Bond
- Thermal Energy
- Intermolecular Forces Vs Thermal Interactions
- The Gaseous State Ex
- The Gas Laws Ex
- Boyle’s Law (Pressure-volume Relationship)
- Charles’ Law (Temperature-volume Relationship)
- Gay Lussac’s Law (Pressure-temperature Relationship)
- Avogadro Law (Volume – Amount Relationship)
- Ideal Gas Equation
- Density And Molar Mass Of A Gaseous Substance
- Dalton’s Law Of Partial Pressures
- Kinetic Molecular Theory Of Gases
- Behavior Of Real Gases: Deviation From Ideal Gas Behavior
- Liquefaction Of Gases
- Liquid State
- Vapor Pressure
- Surface Tension and Viscosity.
Benefits of NCERT Solutions For Class 11 Chemistry Chapter 5
- NCERT is the appropriate guide to start preparing for not only your class11 but also for your 12th and other competitive exams. Additionally, the easy language foundation makes it effortless to understand. Thus students get to complete the subject much before time.
- With the advance completion of the chapters, students can start with questions banks, previous year question paper and sample paper to determine which topic requires attention. Thus offering you ground to work on improvisation before exams.
- Additionally, since you are finishing the chapters before the time you can get to work on rigorous revision sessions as well which is very much helpful in boosting your confidence for the actual exam. Thus increasing your chance of performing brilliantly during the actual exam time.
Access NCERT Solutions For Class 11 Chemistry Chapter 5
Question 1. What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Answer: P1 = 1 bar,P2 = ? V1= 500 dm3 ,V2=200 dm3
As temperature remains constant at 30°C,
P1V1=P2V2
1 bar x 500 dm3 = P2 x 200 dm3 or P2=500/200 bar=2.5 bar
Question 2. A vessel of 120 mL capacity contains a certain amount of gas at 35°C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35°C. What would be its pressure?
Answer: V1= 120 mL, P1=1.2 bar,
V2 = 180 mL, P2 = ?
As temperature remains constant, P1V1 = P2V2
(1.2 bar) (120 mL) = P2 (180mL)
Question 3. Using the equation of state PV = nRT, show that at a given temperature, density of a gas is proportional to the gas pressure P.
Answer: According to ideal gas equation
PV = nRT or PV=nRT/V
Question 4. At 0°C, the density of a gaseous oxide at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Answer: Using the expression, d =MP/RT , at the same temperature and for same density,
M1P1 = M2P2 (as R is constant)
(Gaseous oxide) (N2)
or
M1 x 2 = 28 x 5(Molecular mass of N2 = 28 u)
or M1 = 70u
Question 5. Pressure of l g of an ideal gas A at 27°C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask at same temperature, the pressure becomes 3 bar. Find the relationship between their molecular masses.
Answer: Suppose molecular masses of A and B are MA and MB respectively. Then their number of moles will be
Question 6. The drain cleaner, Drained contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20 °C and one bar will be released when 0.15g of aluminums reacts?
Answer: The chemical equation for the reaction is
2 Al + 2 NaOH + H20 -> 2 NaAl02 + 3H2 (3 x 22400 mL At N.T.P)
2 x 27 = 54 g.
54 g of Al at N.T.P release
H2 gas = 3 x 22400 0.15 g of Al at N.T.P release
Question 7. What will be the pressure exerted by a mixture of 3.2g of methane and 4.4g of carbon dioxide contained in a 9 dm3 flask at 27 °C?
Answer:
Question 8. What will be the pressure of the gas mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in all vessel at 27 °C?
Answer: Calculation of partial pressure of H2 in 1L vessel P1= 0.8 bar,
P2= ? V1= 0.5 L , V2 = 1.0 L
As temperature remains constant, P1V1 = P2V2
(0.8 bar) (0.5 L) = P2 (1.0 L) or P2 = 0.40 bar, i.e., PH2 = 0.40 bar
Calculation of partial pressure of 02 in 1 L vessel
P1‘ V1 = P2‘V2‘
(0.7 bar) (2.0 L) = P2 (1L) or P2‘ = 1.4 bar, i.e.,Po2= 1.4 bar
Total pressure =PHz + PQ2 = 0.4 bar + 1.4 bar = 1.8 bar
Question 9. Density of a gas is found to be 5.46 g/dm3 at 27 °C and at 2 bar pressure. What will be its density at STP?
Answer:
Question 10. 34.05 mL of phosphorus vapor weighs 0.0625 g at 546°C and 1.0 bar pressure. What is the molar mass of phosphorus?
Answer:
Question 11. A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead, he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer, he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?
Answer:
Question 12.Calculate the temperature of 4.0 moles of a gas occupying 5 dm3 at 3.32 bar (R = 0.083 bar dm3 K-1 mol-1)
Answer:
Question 13. Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Answer: Molecular mass of N2 = 28g
28 g of N2 has No. of molecules = 6.022 x 1023 1.4 g of
N2 has No. of molecules = 6.022 x 1023 x 1.4 g/28 g
= 3.011 x 1022 molecules.
Atomic No. of Nitrogen (N) = 7
1 molecule of N2 has electrons = 7 x 2 = 14
3.011 x 1022 molecules of N2 have electrons
= 14 x 3.011 x 1022
= 4.215 x 1023 electrons.
Question 14. How much time would it take to distribute one Avogadro number of wheat grains if 1010 grains are distributed each second ?
Answer:
Question 15. Calculate the total pressure in a mixture of 8g of oxygen and 4g of hydrogen confined in a vessel of l dm3 at 27°C. R = 0.083 bar dm3 K-1 mol-1.
Answer:
Question 16. Pay load is defined as the difference between the mass of the displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at 27°C (Density of air = 1.2 kg m-3 and R = 0.083 bar dm3 K-1 mol-1).
Answer:
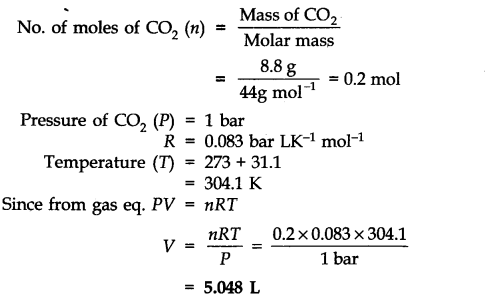
Question 17. Calculate the volume occupied by 8.8 g of CO2 at 31.1 °C and 1 bar pressure. R = 0.083 bar LK-1 mol-1
Answer:
Question 18. 2.9 g of a gas at 95°C occupied the same volume as 0.184 g of hydrogen at 17°C at the same pressure. What is the molar mass of the gas ?
Answer:
Question 19. A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
Answer: As the mixture H2 and O2 contains 20% by weight of dihydrogen, therefore, if H2 = 20g, then O2 = 80g
Question 20. What would be the SI unit for the quantity PV2T2/n?
Answer:
Question 21. In terms of Charles’ law explain why -273°C is the lowest possible temperature.
Answer: At -273°C, volume of the gas becomes equal to zero, i.e., the gas ceases to exist.
Question 22. Critical temperature for CO2 and CH4 are 31.1°C and -81.9°C respectively. Which of these has stronger intermolecular forces and why?
Answer: Higher the critical temperature, more easily the gas can be liquefied, i.e., greater are the intermolecular forces of attraction. Hence, Co2 has stronger intermolecular forces than CH4.
Question 23. Explain the physical significance of vander Waals parameters.
Answer: ‘a’ is a pleasure of the magnitude of the intermolecular forces of attraction, while b is a measure of the effective size of the gas molecules.
MORE QUESTIONS SOLVED
I. Very Short Answer Type Questions
Question 1. What is the value of the gas constant in SI units?
Answer: 8.314 JK-1 mol-1.
Question 2. Define boiling point of a liquid.
Answer: The temperature at which the vapor pressure of a liquid is equal to external pressure is called boiling point of liquid.
Question 3. What is SI unit of (i) Viscosity (ii) Surface tension?
Answer: (i) Unit of viscosity is Nsm-2
(ii) Unit of surface tension is Nm-1
Question 4. What is the effect of temperature on (i) surface tension and (ii) Viscosity?
Answer: (i) Surface tension decreases with increase of temperature.
(ii) Viscosity decreases with increase of temperature.
Question 5. What is the unit of coefficient of viscosity?
Ans. Poise.
Question 6. What do you understand by laminar flow of a liquid?
Answer: The type of flow in which there is regular gradation of velocity in passing from one layer to the next is called laminar flow.
Question 7. What do you mean by compressibility factor?
Answer: The deviation from ideal behaviour can be measured in terms of compressibility factor Z.
Z=PV/nRT
Question 8. What is Boyle Temperature?
Answer: The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure, is called Boyle temperature or Boyle point.
Question 9. What is meant by elastic collision ?
Answer: Collision in which there is no loss of kinetic energy but there is transfer of energy, is called elastic collision.
Question 10. Define critical temperature of gas.
Answer: The temperature above which a gas cannot be liquefied.
Question 11. What are real gases ?
Answer: A gas which can deviate from ideal gas behaviour at higher pressure and lower temperature, is called a real gas.
Question 12. Define an ideal gas.
Answer: A gas that follows Boyle’s law, Charles’ law and Avogadro law strictly, is called an ideal gas.
Question 13. Name four properties of gases.
Answer:
- Gases, have no definite shape and no definite volume.
- There is no force of attraction existing between the molecules of gases.
- Gases are highly compressible.
- Gases ‘can mix evenly and can spread in whole space.
Question 14. State Dalton’s law of partial pressure.
Answer: Daltons’ Law states that, total pressure exerted by the mixture of non-reactive gases is equal to the sum of the partial pressures of individual gases.
Question 15. What do you mean by aqueous tension?
Answer: Pressure exerted by saturated water vapor is called aqueous tension.
Question 16. Give mathematical expression for ideal gas equation.
Answer: PV = nRT
Where R is called Gas constant.
Question 17. Write van der Waals equation for n moles of a gas.
Answer:
Where ‘a’ and ‘V are van derwals constants.
Question 18. How is compressibility factor expressed in terms of molar volume of the real gas and that of the ideal gas?
Answer:
Question 19. Why liquids diffuse slowly as compared to gases?
Answer: In liquids, the molecules are more compact in comparison to gases.
Question 20. What is the effect of temperatures on the vapour pressure of a liquid?
Answer: Vapour pressure increases with rise in temperature.
Question 21. Why falling liquid drops are spherical?
Answer: Because of the property of surface tension, liquid tends to minimise its area.
Here we presented you everything about CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter. Still, if you find any sort of doubts make sure to ask us since are always here to help you out.
FAQ: CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter
Can I download the CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter PDF for free?
Yes, you can download the CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 State Of Matter PDF for free.
What type of questions can be asked from NCERT Solutions For Class 11 Chemistry Chapter 5?
Arithmetic problems on Boyle’s law, Charles’s law, Gay-Lusscac’s law, and Avogadro’s law, and lastly on the partial pressure.
Then there will be questions on critical temperature, pressure, Van der Waals forces and other types of intermolecular forces.
What will you learn in CBSE Class 11 Chemistry Chapter 5?
The state of the matter is the basics that students start learning from class 4 itself. But with time the chapter adds section suiting cognitive progress of the children.
Are these CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 the State Of Matter downloadable on a smartphone?
Yes, CBSE NCERT Solutions For Class 11 Chemistry Chapter 5 the State Of Matter can be downloaded on any device.
What are the Benefits of NCERT Solutions For Class 11 Chemistry Chapter 5?
You can refer to the above article to check the Benefits of NCERT Solutions For Class 11 Chemistry Chapter 5.