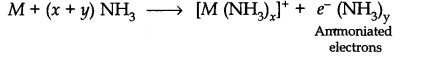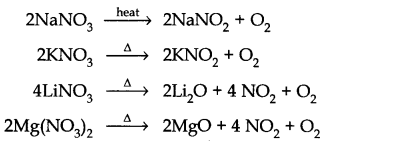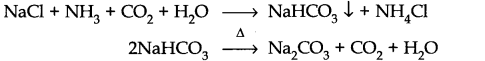NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements: In this NCERT Solutions for Class 11 Chemistry Chapter 10 , we will study the s-block elements of the periodic table. The s-block elements of the periodic table are those elements in which the last electron enters the outermost s-subshell (or s-orbital). Two electrons are the maximum capacity of ns energy subshell.
In this chapter, there are 32 questions in the exercise. The NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements are designed and solved by our chemistry experts. These are the detailed explanation of NCERT textbook questions.
These NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements help students in their preparation of class 11 final examination as well as in the various competitive exams like JEE, NEET, BITSAT etc.
NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements are provided here in pdf format thereby giving better accessibility for all the students. The chapter 10 pdf solutions for Class 11 chemistry contains answers to the questions provided in the textbook along with exemplary problems, MCQ questions from previous year question papers and CBSE sample papers.
By referring to these solutions, students can have a quick revision of the entire topic in a very short interval of time. Solutions for Class 11 Chemistry Chapter 10 serves as important study material for students preparing for examinations and other assignment.
NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2020/10/chapter_10_the_s-block_elements.pdf”, “#example1”);
The NCERT Solutions for Class 11 Chemistry Chapter 10 s block elements PDF is chapter 10 of your prescribed Chemistry textbook. This chapter entails all the vital elements from the s-block of the modern periodic table. It deals with all the properties, structural and functional characteristics, reactions and applications of the elements of the s-block.
Summary About Class 11 Chemistry Chapter 10 The s-Block Elements
An in-depth learning of this chapter from Chemistry will help you to get well-acquainted about Group 1 Elements: Electronic Configuration, Alkali Metals, Ionization Enthalpy, Atomic and Ionic Radii, Hydration Enthalpy, Physical Properties, Chemical Properties, General Characteristics and uses of the Compounds of the Alkali Metals, Oxides and Hydroxides, Halides, Salts of Oxo-Acids,.
Anomalous Properties pf Lithium, difference between Lithium and some other Alkali Metals, important Points of similarities between Lithium and Magnesium, Biological Importance of Sodium and Potassium.
Some Important Compounds of Sodium, Group 2 Elements : Physical Properties, Chemical Properties, the Atomic Electronic and Ionic Radii Configuration, Hydration Enthalpies, Uses, General Characteristics of Compounds of the Alkaline Earth Metals.
Anomalous Behaviour of Beryllium, Some Important Compounds of Calcium and Biological Importance of Magnesium and Calcium, Diagonal Relationship between Aluminium and Beryllium,.
Once you have studied the chapter, you will be able to describe the general characteristics of the alkali metals and their compounds; explain the general characteristics of the alkaline earth metals and their compounds.
manufacture description, properties and uses of industrially important sodium and calcium compounds including Portland cement; appreciate the biological significance of sodium, potassium, magnesium and calcium. Class 11 Chemistry, Chapter 10, s-block elements is an important part of Unit 10.
Get the most accurate and detailed NCERT Solution for Class 11 Chemistry Chapter 10 (The s-Block Elements) solved by expert Chemistry teachers. We provide solutions for questions given in NCERT Solutions for Class 11 Chemistry Chapter 10 Chemistrionicy text-book as per CBSE Board guidelines from the latest NCERT book for Class 11 Chemistry.
The topics and sub-topics in NCERT Solutions for Class 11 Chemistry Chapter 10 .The s-Block Elements are given below.
- Ex 10.1 – GROUP 1 ELEMENTS: ALKALI METALS
- Ex 10.2 – GENERAL CHARACTERISTICS OF THE COMPOUNDS OF THE ALKALI METALS
- Ex 10.2.1 – Oxides and Hydroxides
- Ex 10.2.2 – Halides
- Ex 10.2.3 – Salts of Oxo-Acids
- Ex 10.3 – ANOMALOUS PROPERTIES OF LITHIUM
- Ex 10.4 – SOME IMPORTANT COMPOUNDS OF SODIUM
- Ex 10.5 – BIOLOGICAL IMPORTANCE OF SODIUM AND POTASSIUM
- Ex 10.6 – GROUP 2 ELEMENTS : ALKALINE EARTH METALS
- Ex 10.7 – GENERAL CHARACTERISTICS OF COMPOUNDS OF THE ALKALINE EARTH METALS
- Ex 10.8 – ANOMALOUS BEHAVIOUR OF BERYLLIUM
- Ex 10.9 – SOME IMPORTANT COMPOUNDS OF CALCIUM
- Ex 10.10 – BIOLOGICAL IMPORTANCE OF MAGNESIUM AND CALCIUM.
We cover all exercises in the chapter given below:-
Chapter 10 – 32 Questions with solutions.
Access NCERT Solutions For Class 11 Chemistry Chapter 10
Question 1. What are the common physical and chemical features of alkali metals?
Answer: Physical properties of alkali metals:
- Alkali metals have low ionization enthalpies.
- Alkali metals are highly electropositive in nature.
- Alkali metals exhibit +1 oxidation states in their compounds.
- Alkali metals impart characteristic colours to the flame.
Chemical properties of alkali metals:
- Alkali metals are highly reactive in nature.
- Alkali metals hydroxides are highly basic in nature.
- Alkali metals dissolve in liquid ammonia to form blue and conducting solution.
Question 2. Discuss the general characteristics and gradation in properties of alkaline earth metals.
Answer:
- Atomic size goes on increasing down the group.
- Ionisation energy goes on decreasing down the group.
- They are harder than alkali metals.
- They are less electropositive than alkali metals.
- Electropositive character increases on going down the group.
Question 3. Why are alkali metals not found in nature?
Answer: Alkali metals are highly reactive in nature. That’s why they always exist in combined state in nature.
Question 4. Find out the oxidation state of sodium in Na2O2.
Answer: Let x be the oxidation state of Na in Na2O2 2x + 2 (-1) = 0 2x – 2 = 0 2x = 2 x = +1.
Question 5. Explain why is sodium less reactive than potassium.
Answer: It is because ionization enthalpy ∆Hi of potassium = 419 kJ mol -1.
Ionization enthalpy of sodium = 496 KJ mol. Since Ionization enthalpy of potassium is less than that of sodium, potassium is more reactive than sodium.
Question 6. Compare the alkali metals and alkaline earth metals with respect to (i) ionization enthalpy, (ii) basicity of oxides, (iii) solubility of hydroxides.
Answer: (i) Ionization enthalpy. Because of high nuclear charge the ionization enthalpy
of alkaline earth metals are higher than those of the corresponding alkali metals.
(ii) Basicity of oxides. Basicity of oxides of alkali metals are higher than that of alkaline earth metals.
(iii) Solubility of hydroxides of alkali metals are higher than that of alkaline earth metals. Alkali metals due to lower ionization enthalpy are more electropositive than the corresponding group 2 elements.
Question 7. In what ways lithium shows similarities to magnesium in its chemical behaviour?
Answer:
- Both react with nitrogen to form nitrides.
- Both react with 02 to form monoxides.
- Both the elements have the tendency to form covalent compounds.
- Both can form complex compounds.
Question 8. Explain why can alkali and alkaline earth metals not be obtained by chemical reduction method.
Answer: Alkali and alkaline earth metals are themselves better recusing agents, and reducing agents better than alkali metals are not available. That is why these metals are not obtained by chemical reduction methods.
Question 9. Why are potassium and cesium, rather than lithium used in photoelectric cells?
Answer: Potassium and cesium have much lower ionization enthalpy than that of lithium. As a result, these metals easily emit electrons on exposure to light. Due to this, K and Cs are used in photoelectric cells rather than lithium.
Question 10. When alkali metal dissolves in liquid ammonia, the solution can acquire different colours. Explain the reason for this type of colour change.
Answer: Alkali metals dissolve in liquid ammonia and give deep blue solutions which are conducting in nature because ammoniated electrons absorb energy in the visible region of light and impart blue colour.
Question 11. Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Answer: Due to small size, the ionization enthalpies of Be and Mg are much higher than those of other alkaline earth metals. Therefore, a large amount of energy is needed to excite their valence electron, and that’s why they do not impart colour to the flame.
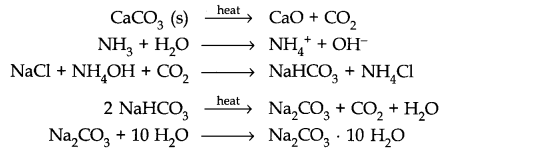
Question 12. Discuss the various reactions that occur in the Solvay process.
Answer:
Question 13. Potassium carbonate cannot be prepared by Solvay process. Why?
Answer: Potassium carbonate being more soluble than sodium bicarbonate does not get precipitated when CO2 is passed through a concentrated solution of KCl saturated with ammonia.
Question 14. Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Answer: Li2CO3 is a covalent compound whereas Na2CO3 is an ionic compound. Therefore, Lattice energy of Na2CO3 is higher than that of Li2CO3. Thus, LiCO3 is decomposed at a lower temperature.
Question 15. Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals.
(a) Nitrates (b) Carbonates (c) Sulphates
Answer: (a) Nitrates of both group 1 and group 2 elements are soluble in water because hydration energy is more than the lattice energy.
Nitrates of both group 1 and group 2 elements are thermally unstable but they decompose differently except LiCO3 e.g.
(b) Carbonates of group 1 elements are soluble in water except Li2CO3 They are also thermally stable except Li2CO3
Group 2 carbonates are insoluble in water because their Lattice energy are higher than hydration energy.
Thermal stability of carbonates of group 2 increases down the group because Lattice energy goes no increasing due to increase in ionic character.
(c) Sulphates of group 1 are soluble in water except Li2SO4. They are thermally stable.
Solubility of sulphates of group 2 decreases down the group because Lattice energy dominates over hydration energy.
Sulphates of group 2 elements are thermally stable and increasing down the group due to increases in Lattice energy.
Question 16. Starting with sodium chloride how would you proceed to prepare.
(i) Sodium metal (ii) Sodium hydroxide
(iii) Sodium peroxide (iv) Sodium carbonate?
Answer: (i) Sodium metal is manufactured by electrolysis of a fused mass of NaCl 40% and CaCl2 60% in Down’s cell at 873 K, using iron as cathode and graphite as anode. Na is liberated at the cathode.
At cathode:
Na+ + e– —–> Na (l)
At anode:
2Cl– (melt) ——-> Cl2 (g) + 2e–.
(ii) Sodium hydroxide is manufactured by electrolysis of an aqueous solution of NaCl (brine) in Castner-Kellner cell.
At cathode:
Na+ + e– —–> Na
2Na + Hg ——->Na – Hg + 2H20
2Na- Hg + 2H20——>2NaOH +H2 +Hg
At anode:
Cl– – e– ——->Cl
Cl + Cl——–>Cl2
(iii) Sodium peroxide:
4Na + 02 2Na2O + 02
(iv)Sodium carbonate is obtained by Solvay ammonia process.
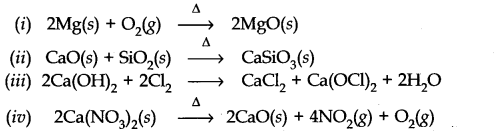
Question 17. What happens when (i) magnesium is burnt in air, (ii) Quick lime is heated with silica (iii) chlorine reacts with slaked lime (iv) calcium nitrate is heated?
Answer:
Question 18. Describe two important uses of each of the following: ,
(i) caustic soda (ii) sodium carbonate (iii) quick lime
Answer: (i) Caustic soda
(a) It is used in the manufacturing of soap paper, artificial silk etc.
(b) It is used in textile industries.
(ii) Sodium carbonate
(a) Used in the softening of water, for laundry and cleaning purposes.
(b) It is used in glass manufacturing.
(iii) Quick lime
(a) It is used in the preparation of bleaching powder.
(b) Used in the purification of sugar and in the manufacturing of cement.
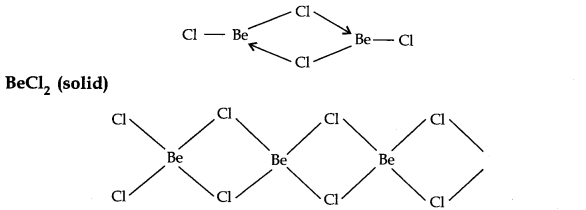
Question 19. Draw the structure of (i) BeCl2 (Vapour), (ii) BeCl2 (solid).
Answer: BeCl2 (Vapour)
In the Vapour state, it exists as a chlorobridged dimer.
Question 20. The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain.
Answer: Since group 1 hydroxides and carbonates due to large size contain higher hydration energy than the lattice energy so, they are easily soluble in water. Whereas, in magnesium and calcium due to small size their lattice energy dominates over hydration energy they are sparingly soluble in water.
Question 21. Describe the importance of the following:
(i) Limestone (ii) Cement (iii) Plaster of Paris.
Answer: Limestone:
- Extensively used in the manufacturing of high quality paper.
- Used as mild abrasive in toothpaste.
- As a filler in cosmetics.
- Used as an antacid.
Cement:
- An important building material.
- Used in concrete and reinforced cement.
Plaster of Paris:
- Used in plasters.
- In dentistry, in ornamental work for making statues.
Question 22. Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
Answer: Due to smallest size, Li+ can polarize water molecules easily than the other alkali metal ions.
Question 23. Why is LiF almost insoluble in water whereas LiCl soluble not only in water but also in acetone?
Answer: It is due to high lattice energy of LiF as compared to LiCl.
LiCl is soluble in water because its hydration energy is higher than its lattice energy.
Question 24. Explain the significance of sodium, potassium, magnesium and calcium in biological fluids.
Answer: Sodium ions:
- Na+ ions participate in the transmission of nerve signals, in regulating the flow of water across cell membranes.
- In the transport of sugars and amino acids into cell.
Potassium ions:
- They activate many enzymes.
- Participate in the oxidation of glucose to produce ATP.
Magnesium ions:
- All enzymes that utilize ATP in phosphate transfer require magnesium as a cofactor.
- Mg is the main pigment for the absorption of light in plants.
Calcium:
- Ca2+ ions are present in bones.
- plays important roles in neuromuscular function.
Question 25. What happens when
(i) Sodium metal is dropped in water?
(ii) Sodium metal is heated in free supply of air?
(iii) Sodium peroxide dissolves in water?
Answer: (i) 2Na + 2H2O ——–> 2NaOH + H2
(ii) 2Na + O2 ———> Na2O2
(iii) Na2O2 + 2H20 ———> 2NaOH + H2O2
Question 26. Comment on each of the following observations:
(a) The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ <K+ < Rb+ < Cs+
(b) Lithium is the only alkali metal to form a nitride directly.
(c) Ee for M2+ (aq) + 2e– —> M(s) (where M = Ca, Sr, or Ba) is nearly constant.
Answer: (a) Smaller the size of the ion, more highly it is hydrated and hence greater is the mass of the hydrated ion and thus the ionic mobility become lesser. The extent of hydration decreases in the order.
Li+ < Na+ <K+ < Rb+ < Cs+
Thus the mobility of Cs+ will be the highest.
(b) Due to its smaller size lithium can form nitride directly.
(c) It is because reduction potential depends upon sublimation energy, ionisation energy and hydration energy. Their resultant is almost constant for these ions.
Question 27. State as to why
(a) a solution of Na2CO3 is alkaline?
(b) alkali metals are prepared by electrolysis of their fused chlorides?
(c) Sodium is found to be move useful than potassium?
Answer: (a) Na2CO3 is a salt of a weak acid, carbonic acid (H2CO3) and a strong base NaOH. Thus it undergoes hydrolysis to produce strong base NaOH and its aqueous solution is alkaline in nature.
Na2CO3(s) + H2O(l)———–>2NaOH
(b) Because the discharge potential of alkali metals is much higher than that of hydrogen, therefore when the aqueous solution of any alkali metal chloride is subjected to electrolysis, H2, instead of the alkali metal, is produced at the cathode. Therefore alkali metals are prepared by electrolysis of their fused chlorides.
(c) Since potassium is move reactive than sodium and it is found in nature to a less extent than Na, sodium is found to be more useful.
Question 28.Write balanced equations for reactions between.
(a) Na2O2 and water
(b) KO2 and water
(c) Na2O and CO2
Answer: (a) Na2O2 + 2H2O ——-> 2Na0H + H2O2
(b) 2KO2 + 2H2O ———-> 2K0H + O2+ H2O2
(c) Na2O+ CO2 ———–>Na2CO3
Question 29. How would you explain the following observations?
(i) BeO is almost insoluble but BeSO4 is soluble in water.
(ii) BaO is soluble but BaSO4is insoluble in water.
(iii) Lil is more soluble than KI in ethanol.
Answer: (i) Lattice energy of BeO is comparatively higher than the hydration energy. Therefore, it is almost insoluble in water. Whereas BeSO4 is ionic in nature and its hydration energy dominates the lattice energy.
(ii) Both BaO and BaSO4 are ionic compounds but the hydration energy of BaO is higher than the lattice energy therefore it is soluble in water.
(iii) Since the size of Li+ ion is very small in comparison to K+ ion, it polarizes the electron cloud of I– ion to a great extent. Thus Lil dissolves in ethanol more easily than the KI.
Question 30. Which of the alkali metal is having least melting point?
(a) Na (b) K (c) Rb (d) Cs
Answer: Size of Cs is the biggest thus, its melting point is the lowest, (d) is correct.
Question 31. Which one of the following alkali metals give hydrated salts?
(a) Li (b) Na (c) K (d) Cs
Answer: Li+ is the smallest. Thus, it has the highest charge density and hence attracts the water molecules more strongly.
Question 32. Which one of the following alkaline earth metal carbonates is thermally most stable?
(a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3
Answer: (d) BaCO3
MORE QUESTIONS SOLVED
NCERT Solutions for Class 11 Chemistry Chapter 10 Very Short Answer Type Questions
Question 1. Name the alkali metal which shows diagonal relationship with magnesium?
Answer: Li.
Question 2. Why alkali and alkaline earth metals cannot be obtained by chemical reduction method?
Answer: Because alkali and alkaline earth metals are themselves stronger reducing agents than the majority of other reducing agents.
Question 3. Name the compounds used for the manufacture of washing soda by Solvay process.
Answer: NaCl, CaCO3 and NH3.
Question 4. Which electrolyte is used to obtain sodium in Castner’s process?
Answer: Fused NaOH.
Question 5. What happens when crystals of washing soda are exposed to air?
Answer: Monohydrate (Na2CO3– H2O) is formed as a result of efflorescence.
Question 6. Name the alkaline earth metals whose salt do not impart colour to a non-luminous flame.
Answer: Beryllium does not impart colour to a non-luminous flame.
Question 7. What is dead burnt plaster?
Answer: It is anhydrous calcium sulphate (CaSO4).
Question 8. What is Quick lime? What happens when it is added to water?
Answer: CaO is quick lime. When it is added to water, Ca(OH)2 is formed.
Question 9. Arrange the following in the increasing order of solubility in water.
MgCl2, CaCl2, SrCl2, BaCl2
Answer: BaCl2 < SrCl2 < CaCl2 <MgCl2
Question 10. Give the chemical formula of Epsom salt.
Answer: MgSO4,7H2O
Question 11. How would you prepare sodium silicate from silica?
Answer:
Question 12. What happens when sodium metal is heated in free supply of air?
Answer: Sodium peroxide is formed.
2Na + O2 ——-> Na2O2
Question 13. What is the general name for elements of group 1 ?
Answer: Alkali metals
We have covered the detailed guide on CBSE NCERT Solutions For Class 11 Chemistry Chapter 10 The S Block Elements. You should have proper CBSE 11th study material to excel at the level of preparation in the correct way. Feel free to ask any questions.
FAQ: CBSE NCERT Solutions For Class 11 Chemistry Chapter 10 The S Block Elements
How NCERT Solutions for Class 11 Chemistry Chapter 10 helpful for Class 11 students?
NCERT Solutions for Class 11 Chemistry Chapter 10 helps students to get a proper grasp of all the concepts of the subjects.
Are these NCERT Solutions for Class 11 Chemistry Chapter 10 available for free download?
Yes, NCERT Solutions for Class 11 Chemistry Chapter 10 are available for free download.
How many questions are there in NCERT Solutions for Class 11 Chemistry Chapter 10?
In NCERT Solutions for Class 11 Chemistry Chapter 10, there are 32 questions in the exercise.
What are the topics included in CERT Solutions for Class 11 Chemistry Chapter 10?
You can refer to the above article to get the CERT Solutions for Class 11 Chemistry Chapter 10.